
Effects of Viscosity on the Isomerization of Calix[4]arene Capped Azobenzene
Student: John Nugent
Major: Chemistry
Advisor: Dr. Paul Bonvallet
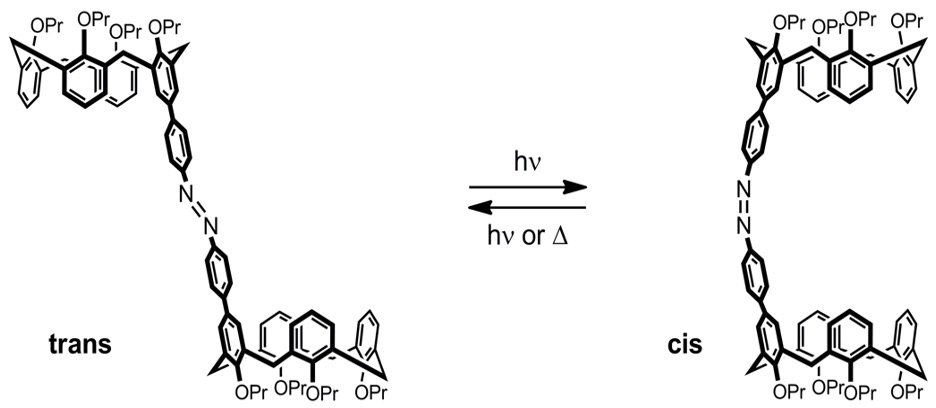
Calix[4]arene capped azobenzene (CCA) takes advantage of the isomerization of azobenzene and the host-guest interactions of calixarenes to act as a molecular capsule that can be opened or closed. The supramolecular capsule can contain positively charged species while in the cis (closed) isomer and release them in the trans (open) isomer. The cis to trans (opening) reaction was the subject of study because it is slow and can be driven by thermal energy. The main goal of this project was to see if increasing the viscosity of the solution would cause the reaction to go slower. The experiments were initially done with azobenzene, then with CCA-1. It was found that there is no relationship between the viscosity of the solution and the rate constant of the cis to trans isomerization in both azobenzene and CCA-1. When the solvent viscosity is increased, the rate of the isomerization remained constant for both.
John will be online to field comments on May 8:
10am-noon EDT (Asia: late evening, PST 6am-8am, Africa/Europe: late afternoon)
Posted in I.S. Symposium, Independent Study on May 5, 2020.
Related Posts
Related Areas of Study
Chemistry
Access to labs, research opportunities, and small classes give chemistry majors lots of options after graduation.
Major Minor

